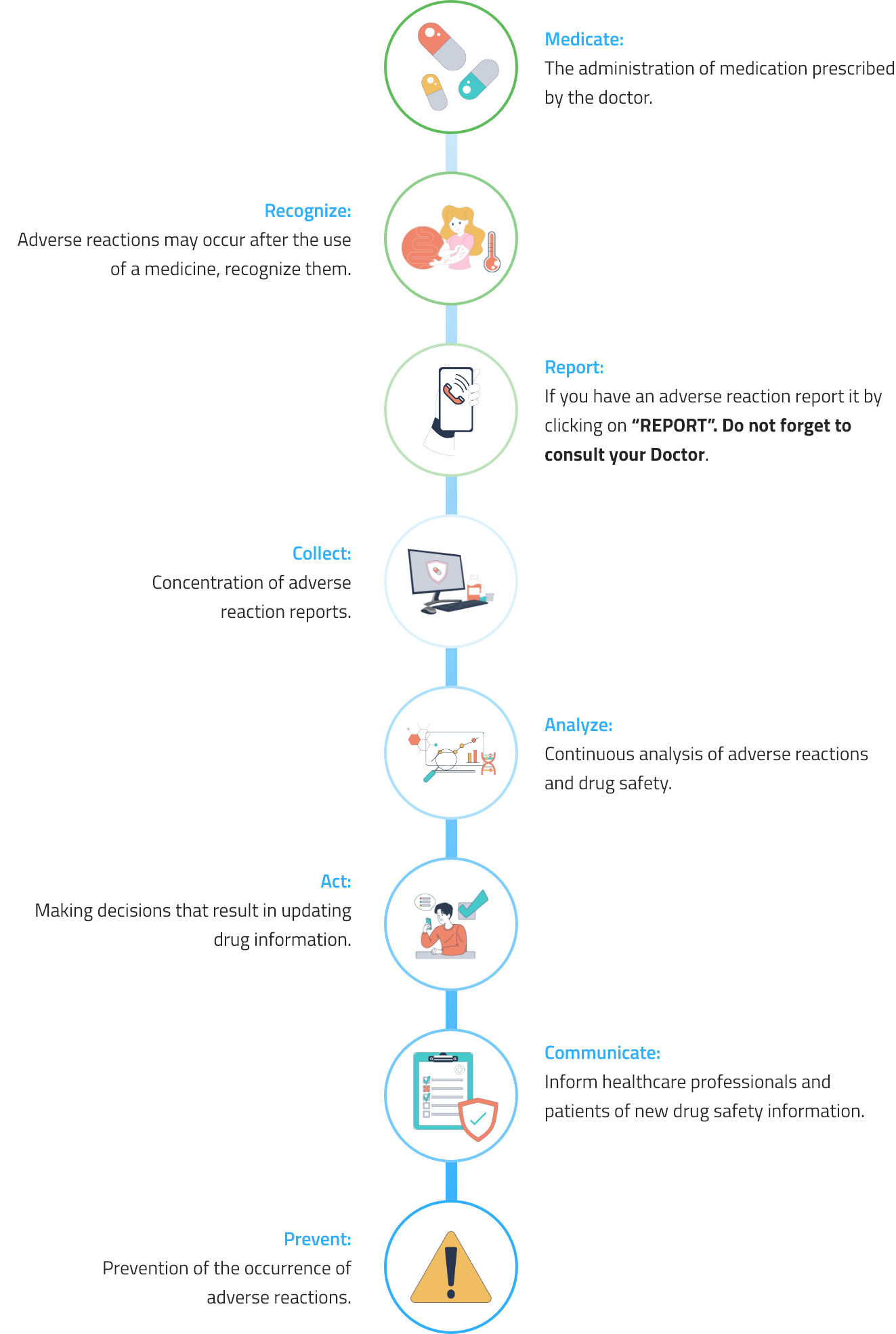
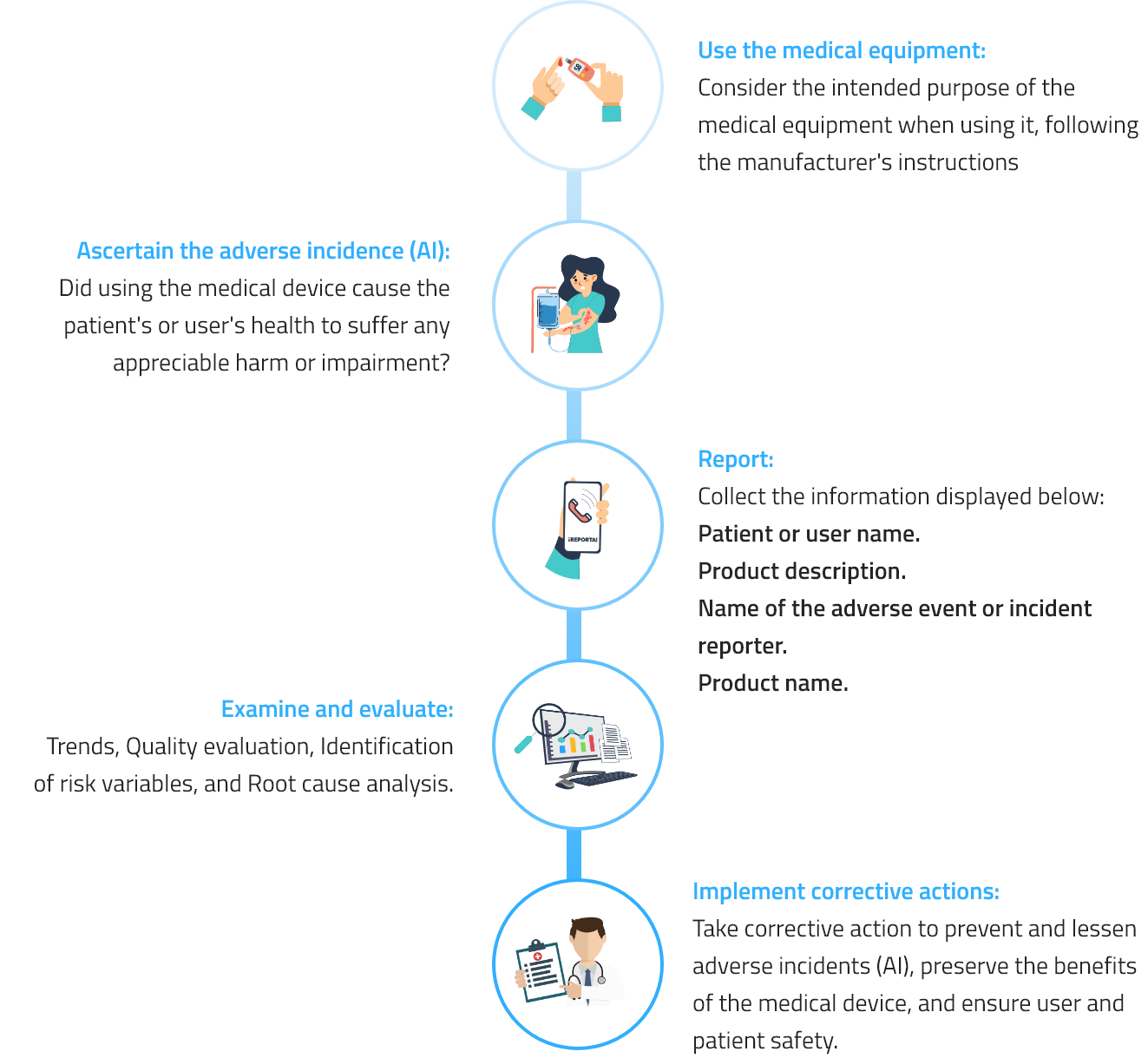
In accordance with current rules, PiSA® Farmacéutica promotes pharmacovigilance and technovigilance, which include ongoing safety monitoring of our medications and medical devices as well as the detection, assessment, understanding, and prevention of any unintended effects or other issues.
The safety of drugs and medical devices is everyone’s responsibility.
PiSA® Farmacéutica, fostering a reporting culture.